Tackling Root Causes: Screening and Addressing Non-Medical Drivers of Health


Scarcity of effective treatments against sepsis is daunting, especially under the contemporary standpoints on antibiotics resistance, entailing the development of alternative treatment strategies. Here, we describe the design and antibiotic adjuvant properties of a new lipopeptide-like pentamer, decanoyl-bis.diaminobutyrate-aminododecanoyl-diaminobutyrate-amide (C10BBc12B), whose sub-maximal tolerated doses combinations with inefficient antibiotics demonstrated systemic efficacies in murine models of peritonitis-sepsis and urinary-tract infections. Attempts to shed light into the mechanism of action using membrane-active fluorescent probes, suggest outer-membrane interactions to dominate the pentamer's adjuvant properties, which were not associated with typical inner-membrane damages or with delayed bacterial growth. Yet, checkerboard titrations with low micromolar concentrations of C10BBc12B exhibited unprecedented capacities in potentiation of hydrophobic antibiotics towards Gram-negative ESKAPE pathogens, with an apparent low propensity for prompting resistance to the antibiotics. Assessment of the pentamer's potentiating activities upon efflux inhibition incites submission of a hitherto unreported, probable action mechanism implicating the pentamer's de-facto capacity to hijack bacterial efflux pumps for boosting its adjuvant activity through repetitive steps including outer-membrane adhesion, translocation and subsequent expulsion.
Sepsis represents one of the most notorious yet ill-treated medical conditions1,2, annually affecting 49 million patients worldwide, with a towering mortality rate of 20%3,4. Effective treatments are urgently needed as current strategies are limited to short-term immunomodulation5,6,7 and broad-spectrum antibiotics administration8,9,10, both of which do not adequately meet the challenge, hence allowing sepsis to persist as leading cause of death3,4. Development of effective treatments is complicated, as they must overcome multiple obstacles pertaining to treatment onset, pathogens diversity, as well as variation in host response1,2. This problem is exacerbated by increased prevalence of antibiotic resistance that further limits current treatments efficacies, particularly with regards to Gram-negative bacteria (GNB), against which, no new antibiotic classes have been successfully developed for over 50 years11. Consequently, exploration of antibiotics combinations is frequently pursued by clinicians, sometimes based on trial and error. Alternatively, immune curbing by agents indifferent to pathogens and/or host variability, may offer a more versatile and potentially preferred treatment strategy. Thus, the ability to sequester contributing factors, such as lipopolysaccharides (LPS) that promote sepsis deterioration to septic-shock, would represent a desirable attribute of such agents.
Cationic antimicrobial peptides (AMPs) are sometimes considered as potential candidates for accomplishing this task. AMPs chemo-physical attributes were evolutionarily designed to selectively target bacterial membranes, including through interactions with anionic moieties of LPS, such as lipid A12,13,14. Nevertheless, their clinical utilization is deemed challenging, namely due to their relatively short half-lives and host-toxicity upon systemic treatments, whereas peptidomimetic approaches are currently believed to minimize such drawbacks, imparting them with robustness and improved chances for successful clinical implementation15. Albeit, those characterized with membranolytic modes of action might in fact achieve the exact opposite outcome, as they too—appear to instigate unregulated LPS release. In this respect, antibiotic adjuvants (namely those using non-specific mechanisms) may provide an attractive treatment alternative16, as they can reinstate an antibiotic's efficacy as well as reduce high-dosage associated toxicity17. Adjuvants can also bestow potency upon Gram-positive-specific hydrophobic antibiotics towards GNB18, specifically when resistance emanates from their low translocation across the outer-membrane (OM)19. Moreover, owing to their mechanistic distinctions from antibiotics, adjuvants can be impervious to the documented resistance mechanisms, emphasizing an inherent advantage for their preferable utilization20. One such group of AMP-mimetics is represented by short lipopeptide-like sequences, composed of amide-linked fatty acids and cationic amino acids21, which, owing to their simple yet modular structure, have recently shown considerable aptitude to facilitate fine-tuning of the chemo-physical properties and subsequent AMP-like biological attributes22,23,24. Here, we sought out to investigate additional new analogs designed to function as adjuvants, through substitution of the cationic amino-acids and then attempted to elucidate the mechanism of action of the most promising emerging analog.
Our previous attempts to design antibiotic adjuvants have focused on short lipopeptide-like sequences corresponding to the pentameric formula AxCCayC; where "Ax" and "ay", respectively represent the N-terminal acyl of length x (number of methyl groups) and an aminoacyl of length y, whereas "C" represents a cationic amino acid. Based on earlier findings24,25, we abstained in the current study from incorporating an exceedingly hydrophobic acyl at the N-terminus, that could generate potent but non-selective antimicrobial lipopeptides, which in turn, might complicate their in-vivo systemic implementation due to toxicity phenomena such as hemolysis and unregulated LPS release. Instead, we used decanoic acid, which emerged from an analogous study26 as potentially embodying the appropriate hydrophobicity for the sequences under present investigation. In parallel, we also attempted to optimize contributions emanating from the cationic residues, by adjusting their side-chain length (Fig. 1a).
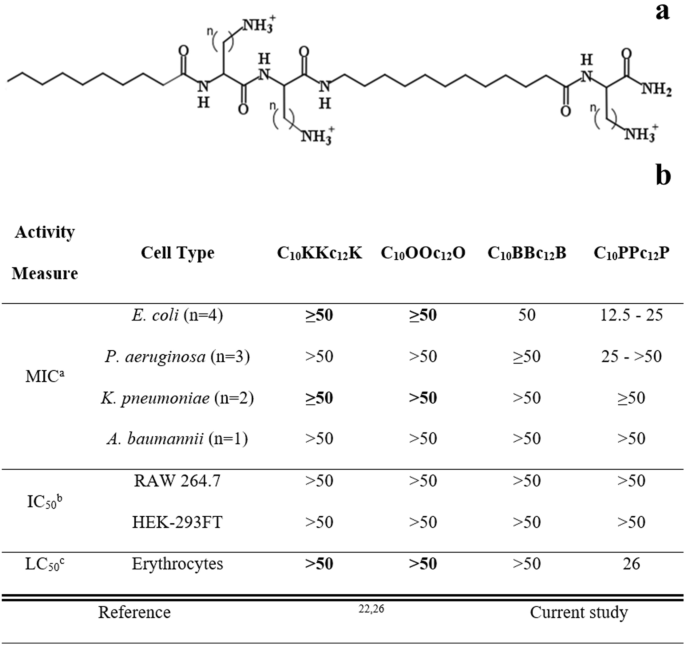
Basic Characterization of Pentamer Analogs. (a) General molecular structure of the A10CCa12C series; where A10 and a12 stand for decanoyl and aminododecanoyl, whereas "C" represents one of the following cationic amino acids: lysine (K), ornithine (O), diaminobutyric acid (B) or diaminopropionic acid (P) where n (methyl groups number on each side chain) equals 4, 3, 2 or 1, respectively. Molecular weights of these analogs are 753, 711, 669 and 627 g/mol, for K, O, B and P, respectively. (b) Cytotoxic activity of pentamer analogs. a, Minimal inhibitory concentrations determined using the broth microdilution method (n, number of tested strains); b, Pentamer concentration causing 50% inhibition of cells respiration, using Alamar blue; c, Pentamer concentration causing 50% hemolysis of murine RBC. Published data appear in bold fonts, shown for comparison purposes.
We first attempted to detect hydrophobicity differences among the cationic analogs by comparing their elution properties, using a C18 reversed-phase HPLC column. However, they all eluted between 45 and 46% hydrophobic solvent (acetonitrile), including upon co-injection of the four analogs, suggesting that the side-chains contribute very little to these molecules chromatographic behavior. This contrasted with previous observations where reducing or increasing the N-terminal acyl length by only 2 methylenes (e.g., C12KKc12K → C10KKc12K → C8KKc12K)24 resulted in substantial hydrophobicity difference (as deduced from the fact that their elution required approximately 5% reduced or increased acetonitrile concentration, respectively). Additionally, assessment of self-assembly tendencies of these lipopeptide analogs (through measuring their light scattering properties) has also demonstrated little dissimilarities in their critical aggregation concentrations, which were all ≥ 100 µM.
Conversely, evaluation of the lipopeptides bioactive properties revealed similarities only between K-, O- and B-based analogs (Fig. 1b), whereas the P-based pentamer presented a divergent activity profile. Namely, C10PPc12P exhibited a hemolytic capacity somewhat more pronounced than that observed with its analogs (i.e., LC50 = 26 versus > 100 µM, respectively). Additionally, when tested against 10 bacterial strains representing the four species of Gram-negative ESKAPE pathogens, the K-, O- and B-based analogs displayed high minimal inhibitory concentrations (MIC ≥ 50 µM), whereas C10PPc12P occasionally presented somewhat lower MIC values. Notably, C10PPc12P presented a lower selectivity profile (i.e., its MIC and LC50 values were comparably scaled) which may be attributed to its side-chain's amine pKa, as it is less likely to be fully protonated at neutral pH (i.e., its β-NH2 group pKa is 6.327 versus at least 10.428, for the three analogs). This could (at least partly) explain its similar potency against RBCs and bacterial cells, particularly since selectivity towards bacterial membranes is significantly charge-driven. Notably, no cytotoxicity towards cell-lines was observed at-least up to 50 µM.
Having established these analogs overall rather poor antibiotic activity against GNB, we next defined their capacities to function as adjuvants that sensitize bacteria to antibiotics. For this purpose, we determined the analogs ability, at a sub-inhibitory concentration (10 µM) to potentiate erythromycin and rifampin (representing two antibiotic families that are normally ineffective against GNB, namely due to their hydrophobicity) using E. coli as representative species. As shown in Table 1, all four analogs presented substantial potentiation capacities, although generally, potency appeared to increase as the cations transitioned from K to B and then decreased with P. Of note, despite the weaker growth-inhibitory activity of C10BBc12B (Fig. 1b), this pentamer exhibited the strongest potentiating activities (Table 1). This is namely evidenced by its lower calculated fractional inhibitory concentration index, compared with the next most potent analog (i.e., 0.2 versus 0.4, respectively for C10BBc12B and C10PPc12P).
Since cyclization of AMPs was found to improve their potency, we similarly produced and tested the corresponding head-to-tail cyclic analogs of the four pentamers. As depicted in Table 1, while maintaining considerable potencies, the cyclic analogs presented substantially lower SF values than their linear counterparts. This finding suggests that structural rigidity contributes to greater antimicrobial potency, whereas flexibility appears to promote stronger adjuvant properties.
Table 1 also compares the pentamers sensitization factors (SF) values with those reported for SPR741, a promising cyclic polymyxin B nonapeptide derivative31 (PMBN) under clinical investigation32. Interestingly, when assessed in another study30 for its capacities to potentiate various antibiotics against 3 GNB species, SPR741 revealed SF values seldom exceeding 1000 folds which, in the case of erythromycin, we found quite similar to those of C10BBc12B (using the same E. coli strain). In the case of rifampin however, although both adjuvants displayed even higher SF values, C10BBc12B revealed to be substantially more potent.
A broader scope of C10BBc12B potentiation capacities is shown in Fig. 2, indicating that the pentamer is endowed with a similarly exquisite capability for sensitizing all four Gram-negative representatives of ESKAPE pathogens tested, as observed with both erythromycin and rifampin. The isobolograms (presented in panels a and b) display geometries indicative of definite synergistic relationships and in most cases, C10BBc12B has potentiated the antibiotics to a point requiring concentrations well-below their respective resistance breakpoints (i.e., 8 and 1 µg/ml respectively for erythromycin and rifampin, as defined against Staphylococci)33. For example, the pentamer reduced erythromycin's inhibitory concentrations against E. coli and K. pneumoniae from 128 and 512 µg/ml to 0.125 and 0.250 µg/ml (i.e., SF values of 1024 and 2048, respectively). Likewise, rifampin's MIC against these species was reduced to below 1 ng/ml, reflecting an increased sensitization efficiency of 65,000 and 130,000 folds, respectively. Such unprecedented values strengthen the proposed stature of C10BBc12B as an exceptionally potent antibiotic adjuvant.
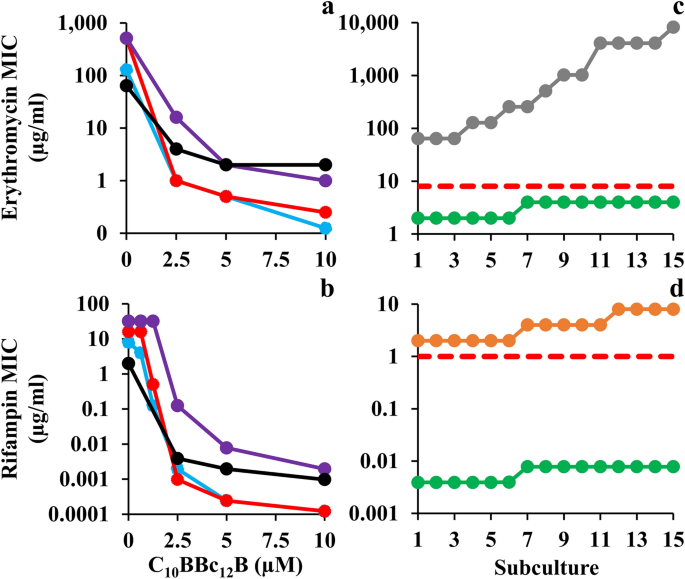
Sensitization of Gram-Negative ESKAPE Pathogens to Antibiotics. (a, b) Isobolograms generated for inhibitory combinations of C10BBc12B with erythromycin and rifampin, respectively. E. coli 25922, cyan; K. pneumoniae 1287, red; P. aeruginosa 27853, purple; A. baumannii 19606, black. (c, d) Antibiotic MIC evolution over 15 consecutive subcultures of A. baumannii 19606, as tested in duplicates, in absence versus in presence of 5 µM C10BBc12B. Erythromycin, gray; rifampin, orange; C10BBc12B, green; resistance breakpoint, dashed red lines.
To assess selective pressure effects on the observed bacterial sensitization to antibiotics, we compared the rates of antibiotic-resistance development by A. baumannii which occupies the top of the urgent category in the latest CDC's antibiotics resistance threats report34. Panels c and d show that in presence of C10BBc12B, the MIC of either antibiotic has not increased by more than 2 folds, which corresponds to the inherent variance of the microdilution technique used during the subculture passages and thus can be regarded as practically unaltered. Conversely, in absence of C10BBc12B, antibiotics' MIC values have increased by 128 and 4 folds, respectively for erythromycin and rifampin (i.e., from 64 to 8,192 µg/ml and from 2 to 8 µg/ml, respectively). This dissimilar increase maybe ascribed to the antibiotics differential modes of action (i.e., bacteriostatic versus bactericidal)35 or to their respective predominant resistance mechanism (i.e., efflux pumps over-expression36 or rpoB mutations37). Regardless, the fact that in presence of C10BBc12B the antibiotics' MIC remained effectively constant, implies that the pentamer has effectively bypassed bacterial aptitudes for developing antibiotic-resistance (at least throughout the assay duration). While the molecular basis is yet to be elucidated, it is worth mentioning that similar assessment was recently reported for SPR74132 on E. coli38. Moreover, as despite the pentamer's greater synergistic potency and the consequently greater evolutionary pressure exerted on resistance development39, resistance has not materialized, thereby highlighting potential advantages of C10BBc12B-based therapies.
Towards determining the potential of C10BBc12B to resolve resilient GNB infections, we used a modified peritonitis-sepsis infection model to verify whether bacteria that were exposed to the pentamer prior to administration to mice, would (in principle) be able to affect the disease course, possibly via LPS neutralization, considering that immune activation by LPS is an underlying factor for sepsis deterioration. Thus, bacteria briefly exposed to C10BBc12B (10 µM in PBS, a concentration known to affect outer-membrane (OM) permeability but does not alter bacterial proliferation rates, as will be shown in Fig. 4) were administered intraperitoneally to neutropenic mice and mortality was recorded for three days thereafter, as compared to control mice infected by the same culture pre-treated with PBS vehicle. As shown in Fig. 3a1, bacteria that were exposed to the pentamer have delayed the onset of mice mortality and reduced the overall lethality rate, thereby reflecting the pentamer's potential ability to interfere with the sepsis process upon treating infected mice, assuming proper pharmacokinetic behavior would be achieved. Encouraged by this observation, we next prepared to assess the pentamer's ability to affect sepsis (using the unmodified peritonitis-sepsis model) upon subcutaneous administration to infected mice of a sub-maximal tolerated dose (MTD).
Thus, MTD determination after administration of increasing doses (i.e., 0, 20 and 40 mg/kg C10BBc12B) to normal mice revealed no apparent physiological signs of distress for any of the mice, from the moment of injection throughout a seven-day monitoring period (i.e., MTD was estimated > 40 mg/kg). This represents a significant improvement compared to its analog C10OOc12O22, for which transient signs of distress were observed in a mouse (score of 3 out of 6) and mortality of another mouse was recorded within an hour after administering the 40 mg/kg dose (i.e., estimated MTD was > 20 but < 40 mg/kg). As the B-based pentamer's MTD is comparable to that reported for the last-resort antibiotic PMB40, this finding suggests that C10BBc12B might offer an improved safety profile given its molecular attributes as small molecule and milder, non-membranolytic putative mechanism of action (as further addressed below).
Next, we evaluated the lipopeptide's ability to affect survival of infected neutropenic mice. Figure 3a2 depicts the mice survival rates upon monotherapy or/and combination-therapies. Thus, while the control mice response to infection has evolved similarly to the experiment shown in panel a1 (i.e., 80% mortality in control mice was reached at two–three days post-infection, using similar inoculum size) mice treated with C10BBc12B or rifampin displayed roughly comparable (40 and 50%, respectively) improved survival rates, as compared to the vehicle control, whereas the combination treatment reached 90% survival. The fact that C10BBc12B showed some efficacy even in absence of rifampin evokes our previous findings22,29 where using a similar infection model and treatment, C10OOc12O has also exhibited significant monotherapy efficacy. While the molecular basis for the pentamers' abilities to prevent mice death in absence of an exogenous antibiotic is yet to be elucidated, we provided various lines of evidence arguing for the possible role played by one or more endogenous antimicrobials that may have substituted for rifampin's role. If that were to be the case, one would be allowed to predict a more potent antimicrobial performance of C10BBc12B in treating infections of wild animals (as opposed to laboratory animals such as the mice used herein) since they are normally endowed with a more robust innate immunity.
Figure 3 (panel a3) provides evidence for considerable systemic efficacy even under harsher infection conditions (i.e., when mice were infected with a higher inoculum size) where lethality of control mice has reached 100% already on day one and mice treated with C10BBc12B or rifampin displayed virtually no efficacy. Under these conditions, the combination treatment managed to increase the survival rates from 0 to 40%, thus implying a synergistic outcome, reminiscent of the synergy observed in terms of SF values (Fig. 2a, b).
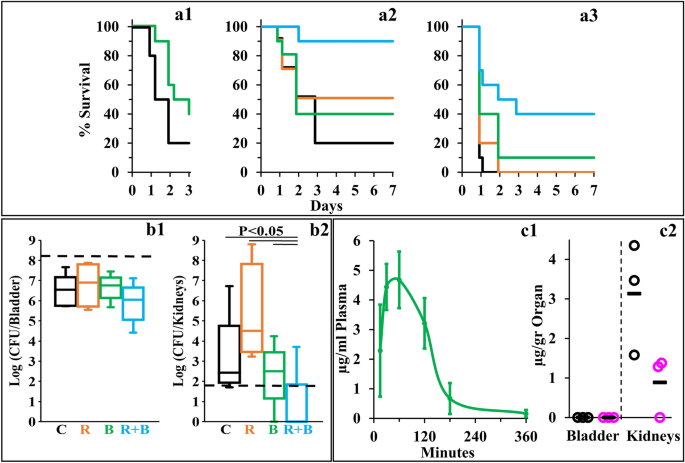
C10BBc12B Properties in Mice Models. (a) Percent survival of neutropenic mice (n = 10 per group) infected with E. coli 25922 using the peritonitis-sepsis infection model. Panel a1 shows survival rates of mice infected with 1.4 × 106 CFU/mouse using a modified model, in which bacteria were pre-treated in-vitro for 15 min with 10 µM C10BBc12B. Bacteria pre-treated with the vehicle control, black; bacteria pre-treated with C10BBc12B, green. Panels a2 and a3 respectively show representative survival rates of mice infected with 1.7 × 106 and 1.9 × 106 CFU/mouse. Vehicle treated control, black; 20 mg/kg rifampin, orange; 12.5 mg/kg C10BBc12B, green; rifampin + C10BBc12B, cyan. (b) Organs bacterial loads in mice (n = 5 per group) infected with 1.2 × 108 CFU/mouse of UPEC CFT073 using the urinary-tract infection model. Box plots in panels b1 and b2 respectively show the CFU counts assessed 24 h post-infection for bladders and kidneys. Vehicle treated control (C) black; 2 mg/kg rifampin (R) orange; 7.5 mg/kg q.i.d. C10BBc12B (B), green; rifampin + C10BBc12B (R + B) cyan. Upper and lower dashed lines respectively represent the inoculum and the limit of detection. (c) Quantification of C10BBc12B in organs of interest, as determined following S.C. administration of 12.5 mg/kg to mice (n = 3 per time point). Panel c1 shows C10BBc12B circulating concentrations in mice plasma. Error bars represent standard deviations. Panel c2 shows the levels detected in mice bladder and kidneys. t = 1 h, black circles; t = 3 h, pink circles; averages, black horizontal bars.
Next, we assessed the pentamer's ability to affect the course of another disease, using the urinary tract infection (UTI) model, where, uro-pathogenic E. coli bacteria were administered by intra-urethral route and the infected mice treated with either C10BBc12B or rifampin or with a combination thereof, and bacterial loads in bladder and kidneys were quantified 24 h post-infection. To determine efficacy, we assessed different treatment regimens, including an initial single dose of 7.5 or 12.5 mg/kg C10BBc12B administered 1 h post infection, but found these regimens to be essentially ineffective, whether administered alone or in combination with rifampin. However, administration of four doses of C10BBc12B within a 24-h period, have generated a significant efficacy upon combination with rifampin. Thus, while no reduction in bacterial load was observed in bladders with any of the tested treatments (Fig. 3b1), the combination treatment has reduced the kidneys bacterial load, as evidenced by complete bacterial eradication (or prevention of colonization altogether) in 80% of the treated mice (Fig. 3b2).
Towards establishing cause and effect relationships, we next attempted to correlate these efficacy outcomes with the circulating pentamer's levels, in the respective tissues of interest. Figure 3c1, c2 present data summarizing the biodistribution analysis showing that C10BBc12B presence overlapped with the efficacies outlined in Fig. 3a, b, respectively. Thus, similarly to its analogs22, the subcutaneous administration of C10BBc12B resulted in a steep increase of its circulating concentrations (reaching its maximum after 30–60 min) followed by a shallower decline that ultimately cleared most of the lipopeptide from the bloodstream after ~ 180 min. Importantly, C10BBc12B maintained antibiotics potentiating concentrations (i.e., ≥ 2.5 µg/ml) for > 2 h post-adjuvant administration. Likewise, quantification of C10BBc12B in bladder and kidneys, revealed negligible amounts in the bladder, whereas the pentamer was readily quantifiable in kidneys for at least 3 h post-inoculation. Therefore, these findings align well with those observed in Fig. 3, in the sense that treatment efficacies are correlated with the adequate pentamer's presence.
Towards better understanding the synergistic mechanism, we compared individual growth kinetics to determine contributions of each antimicrobial and assessed the type and extent of implicated membrane damages, assuming that low OM-permeability underlies the observed antibiotics inefficiencies by impeding their intracellular accumulation. Figure 4a shows that at synergistic concentrations (i.e., 10 µM C10BBc12B and 1 µg/ml erythromycin or 20 ng/ml rifampin) all individual proponents were unable to significantly alter bacterial proliferation (i.e., growth curves obtained for untreated control and individual treatments, were virtually undistinguishable) whereas upon combining treatments, bacterial proliferation was arrested for at least 6 h, followed by bacterial death levels that nearly eradicated the cultures at the 24 h endpoint. Using equivalent conditions, similar growth curves have also characterized K. pneumoniae, P. aeruginosa and A. baumannii (data not shown). Importantly, while sub-inhibitory concentrations of some reported antibiotic potentiators (such as C14(ɷ5)OOc10O23 or PMBN41) have transiently inhibited bacterial growth in absence of antibiotics, the fact that C10BBc12B is devoid of this attribute cements our proposed classification as adjuvant. Also, C10BBc12B combinations with erythromycin or rifampin exhibited similar behaviors—despite the antibiotics distinct modes of action, hence providing additional support to the notion that their potentiation maybe mechanistically related, where the pentamer plays a facilitator role, in both cases. To test this postulate, we next assessed the extent of OM damage using N-phenyl-1-naphthylamine (NPN) fluorescence measurements (Fig. 4). As shown in panel b1, C10BBc12B, like PMB (deemed gold-standard OM-permeabilizer)12,42, increased E. coli's OM permeability to NPN in a dose-dependent manner, albeit in a milder fashion. Validation of these findings is provided by the observation that high concentrations of MgCl2 have attenuated the NPN fluorescence for both agents, correspondingly. Thus, the resulting fluorescence reduction (i.e., almost complete for C10BBc12B and much less for PMB) enforces the concept that C10BBc12B and PMB differ in their affinities towards LPS. This notion was further corroborated using dansyl-PMB (DPMB) competition experiments (Fig. 4 panel b2) by confirming the inferior ability of C10BBc12B to displace the LPS-bound DPMB (the relevance of this affinity difference will be addressed while discussing Fig. 6).
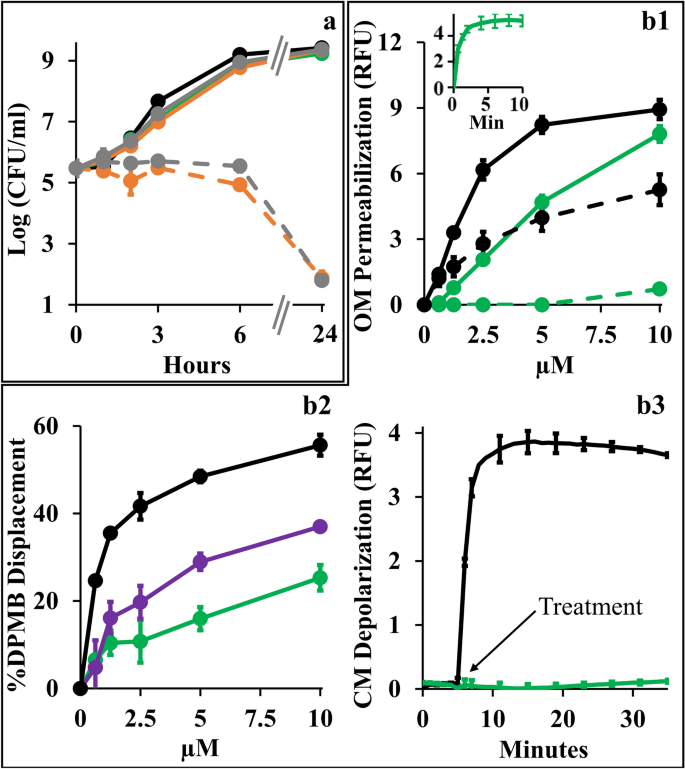
Mechanistic Studies of C10BBc12B Using E. coli 25922. (a) Growth kinetics in presence of single versus combined antimicrobials. Untreated control, black; 10 µM C10BBc12B, green; 1 µg/ml erythromycin, solid gray; 20 ng/ml rifampin, solid orange; 1 µg/ml erythromycin with 10 µM C10BBc12B, dashed gray; 20 ng/ml rifampin with 10 µM C10BBc12B, dashed orange. (b1) OM permeabilization assessed using NPN fluorescence measurements, in presence or absence of 10 mM MgCl2. PMB, solid black; C10BBc12B, solid green; PMB in presence of 10 mM MgCl2, dashed black; C10BBc12B in presence of 10 mM MgCl2, dashed green. Data represent fluorescence after 6 min. Inset shows representative fluorescent signal evolution kinetics for 10 µM C10BBc12B. (b2) Percent displacement of mono-dansylated PMB from E. coli LPS. PMB, black; PMBN, purple; C10BBc12B, green. Data represents results from two independent experiments. (b3) CM depolarization assessed using DiSC3(5) fluorescence measurements. 2.5 µM PMB, black; 10 µM C10BBc12B, green. Arrow denotes the moment of antimicrobials addition after DiSC3(5) baseline signal stabilization. RFU, relative fluorescence units. Error bars represent standard deviations.
Another experiment stressing mechanistic difference(s) between these lipopeptides (C10BBc12B and PMB) is provided through their ability to affect cytoplasmic membrane (CM) permeability. Unlike PMB whose bactericidal activity was associated with a rapid CM-depolarization12 (Fig. 4 panel b3), C10BBc12B was clearly unable to affect the trans-membrane potential (TMP) at the tested concentration range (i.e., up to 10 µM). The fact that a more hydrophilic analog C8BBc12B (data not shown) displayed dose–response depolarization traces that are indistinguishable (i.e., identical to that shown in Fig. 4 panel b3), supports the notion that both derivatives are indeed devoid of some minimal attribute (hydrophobicity?) required for initiating CM-damages.
The lack of observable CM damage upon C10BBc12B interaction with GNB (panel 4b3) is consistent with the kinetics results presented in panel 4a, since if such damage was to occur, some growth delay should have been apparent, as was observed with C14(ɷ5)OOc10O23 and PMBN41 as well as with C10KKc12K24 and to a lesser extent with C10OOc12O22. These observations (i.e., experiments linking OM permeabilization by C10BBc12B to its antibiotics potentiation activity) along with the results establishing the weaker interactions of C10BBc12B with both membranes in comparison with the bactericidal PMB (panels b1–b3) hence render mechanistically puzzling its greater capacity to sensitize GNB to rifampin. Of note, the fact that the displacement profile of C10BBc12B resembled more that of PMBN than PMB (panel b2), suggests that lower LPS affinities promote stronger adjuvant properties.
While C10BBc12B appears to hold mechanistic attributes similar to those of polymyxins, we find that these lipopeptides are distinguishable by various aspects (besides the aforementioned differences) including susceptibility to undergo efflux. When comparatively assessed against the isogenic pair of wild-type E. coli Ag100 and its efflux deficient mutant Ag100a (ΔacrAB) a higher potency of C10BBc12B (eightfold reduction in MIC value) was observed against the mutant strain (Table 2). Similar relationships were recorded with another isogenic pair of Salmonella enterica serovar Typhimurium. These outcomes would sit well with the notion that C10BBc12B is an efflux substrate, very much as previously proposed for its analogs C10KKc12K26 and C10OOc12O29, unlike C14OOc12O23 or C14KKc12K24. Accordingly, the polymyxins (e.g., PMB or colistin), often described as unlikely efflux-substrates43,44,45 are in fact efficient CM-destabilizers that swiftly dissipate the TMP, thereby obstructing the proper proton-dependent function of resistance-nodulation-division (RND) efflux pumps, including the AcrAB-TolC system commonly expressed in Enterobacteriaceae.
Thus, although both PMBN and C10BBc12B may act as antibiotic adjuvants by increasing OM-permeability, we find that they differ in relation to how they affect the TMP and how they are affected by efflux: the former causes TMP dissipation, possibly inactivating proton-dependent efflux (including of itself)47 whereas C10BBc12B appears relatively reluctant to interact with the CM despite its hydrophobic and cationic attributes, thereby sustaining de-facto, its availability for efflux from the periplasm or from superficial adhesion to the CM (as proposed for C10KKc12K26 but not C14KKc12K24).
Another distinguishing aspect emanated from the lipopeptides divergent behavior in presence of efflux pump inhibitors (EPIs). Since PMBN was reported to synergize with EPIs in potentiating antibiotics activity41, we verified whether C10BBc12B potentiation could be similarly enhanced in presence of EPIs especially since, in the pentamer's case, efflux function stands presumably unaffected. However, introduction of EPIs appears to rather dampen the pentamer's capacity for antibiotics potentiation. Thus, towards isolating relationships between C10BBc12B, EPI and efflux machinery, we used rifampin (instead of erythromycin) owing to its non-susceptibility for efflux by RND pumps48 (and in Table 2). Figure 5a shows that C10BBc12B SF values, as determined in absence of the EPI phenylalanine-arginine β-naphthylamide (PAβN)49,50, were in fact greater than those obtained in its presence, thereby suggesting an antagonistic relationship between C10BBc12B and PAβN or even another EPI49,51, unlike PMBN's41. It is ought to be mentioned that while functioning as efflux pumps inhibitors, these EPIs 52,53 were additionally reported to increase OM permeability (as might also be inferred from our observations, as depicted in Fig. 5a). Unfortunately therefore, as this dual mechanism of action of PAβN (i.e., pump inhibitor and OM permeabilizer), complicates the assignment of either one of its functions to the antagonistic relationship with C10BBc12B, we attempted to circumvent this challenge by using an alternative approach for limiting efflux function, and depleted the fuel required for proper function of RND pumps (the proton motive force) by introducing the ionophore carbonyl cyanide m-chlorophenyl hydrazone (CCCP)54,55. As shown in Fig. 5b, CCCP has diminished the OM-permeabilization exerted by C10BBc12B. Importantly, NPN fluorescent signals obtained with the untreated control and with the CCCP-treated control (without lipopeptide) were practically identical, indicating that CCCP affected the proton motive force, without compromising OM integrity. These findings therefore support a scenario in which proper efflux function could boost OM-permeabilization by C10BBc12B.
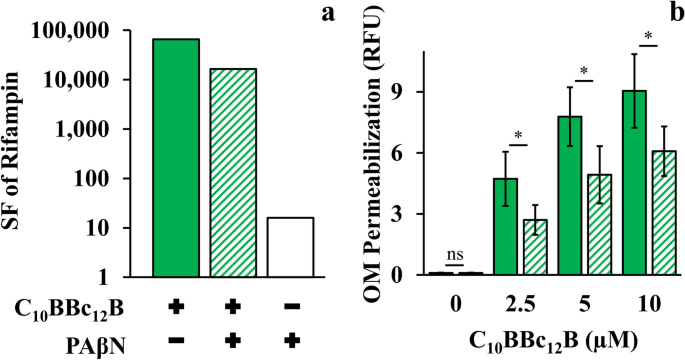
Efflux Role in C10BBc12B OM-Permeabilization Using E. coli 25922. (a) Sensitization factors (SF) determined for rifampin in presence of 10 µM C10BBc12B, solid green bars; 10 µM C10BBc12B + 5 µg/ml PAβN, striped green bars; 5 µg/ml PAβN, white bars. (b) OM permeabilization assessed using a modified NPN assay, following 5 min incubation with 100 µM CCCP. C10BBc12B, solid green bars, C10BBc12B in presence of 100 µM CCCP, striped green bars. Data represent fluorescence after 1 min. Asterisks denote P < 0.05; ns, not significant. RFU, relative fluorescence units. Error bars represent standard deviations.
The data collected so far (particularly observations detailed in Figs. 4 and 5) insinuate mechanistic aspects that might explain the extraordinary potentiation capacities of C10BBc12B. In our understanding, they could be rationalized to reflect a cyclic scenario consisting of three main steps, as depicted in Fig. 6.
Adhesion: As described for PMB by the self-promoted uptake theory56, electrostatic attraction would initially lead to C10BBc12B adherence to anionic OM components (namely LPS). This interaction effectively displaces LPS-bound bivalent cations (that normally limit lateral motion by "gluing" adjacent lipid-A molecules) thereby expanding the monolayer fluidity. This effect is likely exacerbated by the lipopeptide's larger molecular volume that further increases the intermolecular distances, thereby leading to transient crack formations, through which hydrophobic molecules may sift inwards.
Translocation: These presumably short bursts of membrane disruption events could nevertheless eventually mount to the translocation of otherwise excluded hydrophobic compounds (exemplified in this work by rifampin and erythromycin) thereby facilitating their interaction with cytoplasmic targets. Concomitantly, this resultant OM-permeabilization also promotes the translocation of additional C10BBc12B molecules.
Expulsion: After translocating into the periplasm, the pentamer's fate can theoretically follow several routes, including exit the periplasm autonomously (unlikely, since it would require diffusion opposing the lipopeptide's concentration gradient), interaction with periplasmic constituents including the CM outer leaflet and/or expulsion by efflux. Likely therefore, at this stage, C10BBc12B would be simultaneously attracted to both the CM anionic phospholipids and to the efflux pump hydrophobic pocket57,58,59. Here too, the lipopeptide's moderate hydrophobicity might serve as a key determinant for the subsequent outcome, as it was shown that hydrophobic analogs (such as C14KKc12K24) might escape extrusion by efflux pumps via strong interactions and deep insertion within the CM60,61, where they would instigate damages to various extents24,62. In this respect, the pentamer's hydrophobicity appears to be sufficiently low so as to restrict itself to a superficial CM-adherence at most, which would limit its escape from efflux (or at least prolong its unbound state in the periplasm, which increases its chances for expulsion). Notably, the intact TMP sustains the pump's ability to expel the pentamer, which now would be able to re-adhere to the OM and repeat the cycle all over again.