Tackling Root Causes: Screening and Addressing Non-Medical Drivers of Health


Bacteria use molecular machines to move proteins, including toxins, across cell membranes. M. tuberculosis, which kills more than 1 million people a year, uses the ESX-4 type VII secretion system to transports its potent exotoxin.
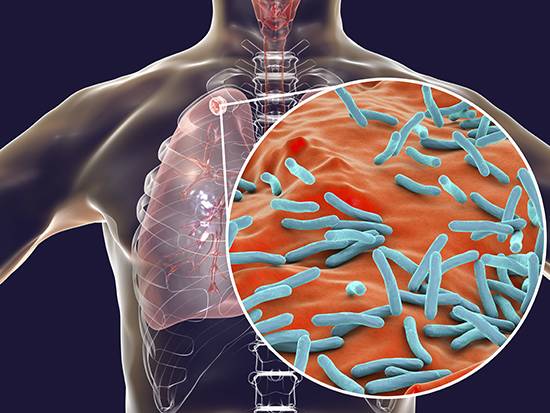 Bacteria use molecular machines to move proteins, including toxins, across cell membranes. M. tuberculosis, which kills more than 1 million people a year, uses the ESX-4 type VII secretion system to transports its potent exotoxin.Six years ago, Michael Niederweis, Ph.D., described the first known toxin of the deadly pathogen Mycobacterium tuberculosis (Mtb), an exotoxin that had gone undetected for 132 years.
Bacteria use molecular machines to move proteins, including toxins, across cell membranes. M. tuberculosis, which kills more than 1 million people a year, uses the ESX-4 type VII secretion system to transports its potent exotoxin.Six years ago, Michael Niederweis, Ph.D., described the first known toxin of the deadly pathogen Mycobacterium tuberculosis (Mtb), an exotoxin that had gone undetected for 132 years.
Now Niederweis and colleagues at the University of Alabama at Birmingham describe the mechanism of secretion and trafficking of that toxin, TNT, which is the major cytotoxicity factor for the pathogen that infects 9 million people a year and kills more than 1 million.
Their report, published in Nature Communications, identifies key secretion systems used by Mtb in pathogenesis, which makes them candidate targets for therapeutics.
When Mtb bacteria are inhaled into a human lung, they are engulfed by host lung macrophages and trapped inside intracellular vesicles called phagosomes. Mtb prevents the phagosome from merging with a lysosome, which otherwise would kill the bacteria. Instead, Mtb safely grows inside the phagosomes, then breaks out of the phagosomes into the cytosol of the macrophage, and it uses the TNT toxin to kill the macrophage by necroptosis, releasing the bacteria to infect other cells.
Exotoxin export from Mtb or similar bacteria face two barriers — first, the toxin protein needs to be transported across the bacteria's cytoplasmic membrane, and then, it needs to be transported across the bacteria's outer membrane. To do this, bacteria employ a wide variety of specialized secretion systems — essentially molecular machines that recognize and transport the bacterial toxins or other proteins involved in a variety of bacterial functions, such as adherence or scavenging.
Toxin secretion mechanisms have been described in almost all major bacterial pathogens, but there has been one notable exception — Mtb.
 Michael Niederweis, Ph.D., Now, Niederweis, David Pajuelo, Ph.D., Uday Tak, Ph.D., Lei Zhang, Ph.D., UAB Department of Microbiology, have used a comprehensive genetic analysis to learn which of the five type VII secretion systems in Mtb is used for TNT secretion. They also discovered which three secretion systems are essential, acting in concert, to permeabilize the phagosome membrane. That sieve-like damage allows TNT to enter the macrophage cytosol, where it depletes NAD+to induce necroptotic death.
Michael Niederweis, Ph.D., Now, Niederweis, David Pajuelo, Ph.D., Uday Tak, Ph.D., Lei Zhang, Ph.D., UAB Department of Microbiology, have used a comprehensive genetic analysis to learn which of the five type VII secretion systems in Mtb is used for TNT secretion. They also discovered which three secretion systems are essential, acting in concert, to permeabilize the phagosome membrane. That sieve-like damage allows TNT to enter the macrophage cytosol, where it depletes NAD+to induce necroptotic death.
The five type VII secretion systems in Mtb are known as ESX-1, ESX-2, ESX-3, ESX-4 and ESX-5. Each of these secretion systems is a complex of between seven and more than 15 proteins that form the molecular machinery. Up to now, no roles were known for ESX-2 and ESX-4.
TNT begins its journey as a part of a larger protein, CpnT. Only when CpnT reaches the outer membrane does the toxic TNT break away from the larger protein. Niederweis and colleagues found that both export to the cell surface and secretion of CpnT/TNT into the cytosol of macrophages infected with Mtb require the ESX-4 system. This is the first known molecular function for the ESX-4 system in Mtb.
While it was known that ESX-1 participates in permeabilizing the phagosome membrane, the UAB researchers surprisingly found that ESX-2 and ESX-4 are also required to act in concert with ESX-1 to rupture the phagosomal membrane and enable trafficking of TNT into the cytosol of Mtb-infected macrophages.
"Thus, our study identifies not only the system required for the secretion of the only known exotoxin of Mtb," said Niederweis, "but also establishes new molecular roles for the two previously uncharacterized type VII secretion systems, ESX-2 and ESX-4, in phagosomal rupture, a critical step in Mtb pathogenesis."
In the Nature Communications paper, the researchers also propose a model for TNT production, export, secretion and trafficking by Mtb, and they point out the steps of this process that are still unclear.
"The paradigm-changing discovery that both the ESX-2 and ESX-4 systems need to act in concert with the ESX-1 system to permeabilize the phagosomal membrane raises important questions regarding the molecular mechanism of this process and the regulation of these activities," Niederweis said. "This study presents a major advancement in our understanding of protein secretion and trafficking by Mtb and will certainly stimulate further research in these important areas of Mtb biology."
Co-authors with Niederweis, Pajuelo, Tak and Zhang in the study, "Toxin secretion and trafficking by Mycobacterium tuberculosis," are Olga Danilchanka, UAB Department of Microbiology, and Anna D. Tischler, Department of Microbiology and Immunology, University of Minnesota.
Support came from National Institutes of Health grant AI121354.
At UAB, Niederweis holds the Endowed Professorship in Bacteriology.
Comments
Post a Comment